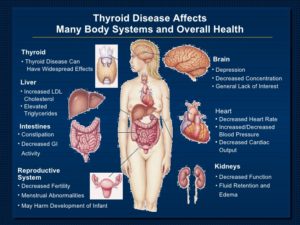
Diagnostic Tools for Hypothyroidism:
History: The doctor will take a detailed history, evaluating both past and present medical problems. If the patient is younger than 20 or older than 70 years, there is increased likelihood that a nodule is cancerous. Similarly, the nodule is more likely to be cancerous if there is any history of radiation exposure, difficulty swallowing, or a change in the voice. It was actually customary to apply radiation to the head and neck in the 1950s to treat acne! Significant radiation exposures include the Chernobyl and Fukushima disasters. Although women tend to have more thyroid nodules than men, the nodules found in men are more likely to be cancerous. Despite its value, the history cannot differentiate benign from malignant nodules. Thus, many patients with risk factors uncovered in the history will have benign lesions. Others without risk factors for malignant nodules may still have thyroid cancer.
Physical examination: The physician should determine if there is one nodule or many nodules, and what the remainder of the gland feels like. The probability of cancer is higher if the nodule is fixed to the surrounding tissue (unmovable). In addition, the physical exam should search for any abnormal lymph nodes nearby that may suggest the spread of cancer. In addition to evaluating the thyroid, the physician should identify any signs of gland malfunction, such as thyroid hormone overproduction (hyperthyroidism) or underproduction (hypothyroidism).
Blood tests: Initially, blood tests should be done to assess thyroid function. These tests include:
- The free T4 and thyroid stimulating hormone (TSH) levels. Elevated levels of the thyroid hormones T4 or T3 in the context of suppressed TSH suggests hyperthyroidism
- Reduced T4 or T3 in the context of high TSH suggests hypothyroidism
- Antibody titers to thyroperoxidase or thyroglobulin may be useful to diagnose autoimmune thyroiditis
- (for example, Hashimoto’s thyroiditis).
- If surgery is likely to be considered for treatment, it is strongly recommended that the physician als determine the level of thyroglobin. Produced only in the thyroid hormone in the blood. Thyroglobulin carries thyroid hormone in the blood. Thyroglobulin levels should fall quickly within 48 hours in the thyroid gland is completely remobed. If thyroglobulin levels start to climb.
Ultrasonography: A physician may order an ultrasound examination of the thyroid to:
- Detect nodules that are not easily felt
- Determine the number of nodules and their sizes
- Determine if a nodule is solid or cystic
- Assist obtaining tissue for diagnosis from the thyroid with a fine needle aspirate (FNA)
Despite its value, the ultrasound cannot determine whether a nodule is benign or cancerous.
Radionuclide scanning: Radionuclide scanning with radioactive chemicals is another imaging technique a physician may use to evaluate a thyroid nodule. The normal thyroid gland accumulates iodine from the blood and uses it to make thyroid hormones. Thus, when radioactive iodine (123-iodine) is administered orally or intravenously to an individual, it accumulates in the thyroid and causes the gland to “light up” when imaged by a nuclear camera (a type of Geiger counter). The rate of accumulation gives an indication of how the thyroid gland and any nodules are functioning. A “hot spot” appears if a part of the gland or a nodule is producing too much hormone. Non-functioning or hypo-functioning nodules appear as “cold spots” on scanning. A cold or non-functioning nodule carries a higher risk of cancer than a normal or hyper-functioning nodule. Cancerous nodules are more likely to be cold, because cancer cells are immature and don’t accumulate the iodine as well as normal thyroid tissue. However, cold spots can also be caused by cysts. This makes the ultrasound a much better tool for determining the need to do an FNA.
Fine needle aspiration: Fine needle aspiration (FNA) of a nodule is a type of biopsy and the most common, direct way to determine what types of cells are present. The needle used is very thin. The procedure is simple and can be done in an outpatient office, and anesthetic is injected into tissues traversed by the needle. FNA is possible if the nodule is easily felt. If the nodule is more difficult to feel, fine needle aspiration can be performed with ultrasound guidance. The needle is inserted into the thyroid or nodule to withdraw cells. Usually, several samples are taken to maximize the chance of detecting abnormal cells. These cells are examined microscopically by a pathologist to determine if cancer cells are present. The value of FNA depends upon the experience of the physician performing the FNA and the pathologist reading the specimen. Diagnoses that can be made from FNA include:
- Benign thyroid tissue (non-cancerous) can be consistent with Hashimoto’s thyroiditis, a colloid nodule, or a thyroid cyst. This result is reported from approximately 60% of biopsies.
- Cancerous tissue (malignant) can be consistent with diagnosis of papillary, follicular, or medullary cancer. This result is reported from approximately 5% of biopsies. The majority of these are papillary cancers.
- Suspicious biopsy can show a follicular adenoma. Though usually benign, up to 20% of these nodules are found ultimately to be cancerous.
- Non-diagnostic results usually arise because insufficient cells were obtained. Upon repeat biopsy, up to 50% of these cases can be distinguished as benign, cancerous, or suspicious.
One of the most difficult problems for the pathologist is to be confident that a follicular adenoma – usually a benign nodule – is not a follicular cell carcinoma or cancer. In these cases, it is up to the physician and the patient to weigh the option of surgery on a case-by-case basis, with less reliance on the pathologist’s interpretation of the biopsy. It is also important to remember that there is a small risk (3%) that a benign nodule diagnosed by FNA may still be cancerous. Thus, even benign nodules should be followed closely by the patient and physician. Another biopsy may be necessary, especially if the nodule is growing. Most thyroid cancers are not very aggressive; that is, they do not spread rapidly. The exception is poorly differentiated (anaplastic) carcinoma, which spreads rapidly and is difficult to treat.
Treatment for Hypothyroidism:
Hypothyroidism happens when the thyroid gland doesn’t make enough hormones. Conditions or problems that can lead to hypothyroidism include:
- Autoimmune disease. The most common cause of hypothyroidism is an autoimmune disease called Hashimoto’s disease. Autoimmune diseases happen when the immune system makes antibodies that attack healthy tissues. Sometimes that process involves the thyroid gland and affects its ability to make hormones.
- Thyroid surgery. Surgery to remove all or part of the thyroid gland can lower the gland’s ability to make thyroid hormones or stop it completely.
- Radiation therapy. Radiation used to treat cancers of the head and neck can affect the thyroid gland and lead to hypothyroidism.
- Thyroiditis. Thyroiditis happens when the thyroid gland becomes inflamed. This may be due to an infection. Or it can result from an autoimmune disorder or another medical condition affecting the thyroid. Thyroiditis can trigger the thyroid to release all of its stored thyroid hormone at once. That causes a spike in thyroid activity, a condition called hyperthyroidism. Afterward, the thyroid becomes underactive.
- Medicine. A number of medicines may lead to hypothyroidism. One such medicine is lithium, which is used to treat some psychiatric disorders. If you’re taking medicine, ask your heath care provider about its effect on the thyroid gland.
Less often, hypothyroidism may be caused by:
- Problems present at birth. Some babies are born with a thyroid gland that doesn’t work correctly. Others are born with no thyroid gland. In most cases, the reason the thyroid gland didn’t develop properly is not clear. But some children have an inherited form of a thyroid disorder. Often, infants born with hypothyroidism don’t have noticeable symptoms at first. That’s one reason why most states require newborn thyroid screening.
- Pituitary disorder. A relatively rare cause of hypothyroidism is the failure of the pituitary gland to make enough thyroid-stimulating hormone (TSH). This is usually because of a noncancerous tumor of the pituitary gland.
- Pregnancy. Some people develop hypothyroidism during or after pregnancy. If hypothyroidism happens during pregnancy and isn’t treated, it raises the risk of pregnancy loss, premature delivery and preeclampsia. Preeclampsia causes a significant rise in blood pressure during the last three months of pregnancy. Hypothyroidism also can seriously affect the developing fetus.
- Not enough iodine. The thyroid gland needs the mineral iodine to make thyroid hormones. Iodine is found mainly in seafood, seaweed, plants grown in iodine-rich soil and iodized salt. Too little iodine can lead to hypothyroidism. Too much iodine can make hypothyroidism worse in people who already have the condition. In some parts of the world, it’s common for people not to get enough iodine in their diets. The addition of iodine to table salt has almost eliminated this problem in the United States.
- If you have had radiation therapy and have hypothyroidism, or if your thyroid gland has been removed, you will most likely need treatment from now on. If your hypothyroidism is caused by Hashimoto’s thyroiditis, you might also need treatment from now on. Sometimes, thyroid gland function returns on its own in Hashimoto’s thyroiditis.
- If a serious illness or infection triggered your hypothyroidism, your thyroid function most likely will return to normal when you recover.
- Some medicines may cause hypothyroidism. Your thyroid function may return to normal when you stop the medicines.
- If you have mild (subclinical) hypothyroidism, you may not need treatment but should be watched for signs of hypothyroidism getting worse. You and your doctor will talk about the pros and cons of taking medicine to treat your mild hypothyroidism. The dose of thyroid medicine must be watched carefully in people who also have heart disease, because too much medicine increases the risk of chest pain (angina) and irregular heartbeats (atrial fibrillation).
*****1.)If you have severe hypothyroidism by the time you are diagnosed, you will need immediate treatment. Severe, untreated hypothyroidism can cause myxedema coma, a rare, life-threatening condition.
2.)Treatment during pregnancy is especially important, because hypothyroidism can harm the developing fetus.
- If you develop hypothyroidism during pregnancy, treatment should be started immediately. If you have hypothyroidism before you become pregnant, your thyroid hormone levels need to be checked to make sure that you have the right dose of thyroid medicine. During pregnancy, your dose of medicine may need to be increased by 25% to 50%.
- You are likely to need treatment for hypothyroidism from now on. As a result, you need to take your medicine as directed. For some people, hypothyroidism gets worse as they age and the dosage of thyroid medicine may have to be increased gradually as the thyroid continues to slow down.
- Most people treated with thyroid hormone develop symptoms again if their medicine is stopped. If this occurs, medicine needs to be restarted.
Treatment for Hyperthyroidism:
If your symptoms bother you, your doctor may give you pills called beta-blockers. These can help you feel better while you and your doctor decide what your treatment should be. Hyperthyroidism can lead to more serious problems. So even if your symptoms do not bother you, you still need treatment.
The diagnostic workup for hyperthyroidism includes measuring thyroid-stimulating hormone, free thyroxine (T4), and total triiodothyronine (T3) levels to determine the presence and severity of the condition, as well as radioactive iodine uptake and scan of the thyroid gland to determine the cause.
There are several treatments available for hyperthyroidism. The best approach for you depends on your age and health. The underlying cause of hyperthyroidism and how severe it is make a difference too. Your personal preference also should be considered as you and your health care provider decide on a treatment plan. Treatment may include:
- Anti-thyroid medicine. These medications slowly ease symptoms of hyperthyroidism by preventing the thyroid gland from making too many hormones. Anti-thyroid medications include methimazole and propylthiouracil. Symptoms usually begin to improve within several weeks to months.Treatment with anti-thyroid medicine typically lasts 12 to 18 months. After that, the dose may be slowly decreased or stopped if symptoms go away and if blood test results show that thyroid hormone levels have returned to the standard range. For some people, anti-thyroid medicine puts hyperthyroidism into long-term remission. But other people may find that hyperthyroidism comes back after this treatment.Although rare, serious liver damage can happen with both anti-thyroid medications. But because propylthiouracil has caused many more cases of liver damage, it’s generally used only when people can’t take methimazole. A small number of people who are allergic to these medicines may develop skin rashes, hives, fever or joint pain. They also can raise the risk of infection.
- Beta blockers. These medicines don’t affect thyroid hormone levels. But they can lessen symptoms of hyperthyroidism, such as a tremor, rapid heart rate and heart palpitations. Sometimes, health care providers prescribe them to ease symptoms until thyroid hormones are closer to a standard level. These medicines generally aren’t recommended for people who have asthma. Side effects may include fatigue and sexual problems.Regardless of the cause of hyperthyroidism, the adrenergic symptoms are controlled by beta blockers Propranolol has the theoretical advantage of also inhibiting 5′-monodeiodinase, thus blocking peripheral conversion of T4 to T3.
- Radioiodine therapy. The thyroid gland takes up radioiodine. This treatment causes the gland to shrink. This medicine is taken by mouth. With this treatment, symptoms typically lessen within several months. This treatment usually causes thyroid activity to slow enough to make the thyroid gland underactive. That condition is hypothyroidism. Because of that, over time, you may need to take medicine to replace thyroid hormones.
- Radioactive iodine and antithyroid medicine are the treatments doctors use most often. The best treatment for you will depend on a number of things, including your age. Some people need more than one kind of treatment.
- Thyroidectomy. This is surgery to remove part of or all of the thyroid gland. It is not used often to treat hyperthyroidism. But it may be an option for people who are pregnant. It also may be a choice for those who can’t take anti-thyroid medicine and don’t want to or can’t take radioiodine therapy.Risks of this surgery include damage to the vocal cords and parathyroid glands. The parathyroid glands are four tiny glands on the back of the thyroid. They help control the level of calcium in the blood.People who have a thyroidectomy or radioiodine therapy need lifelong treatment with the medicine levothyroxine (Levoxyl, Synthroid, others). It supplies the body with thyroid hormones. If the parathyroid glands are removed during surgery, medicine also is needed to keep blood calcium in a healthy range.
Thyroid eye disease
If you have thyroid eye disease, you may be able to manage mild symptoms with self-care steps, such as artificial tear drops and lubricating eye gels. Avoiding wind and bright lights can help too.
More-severe symptoms may need treatment with medicine called corticosteroids, such as methylprednisolone or prednisone. They can lessen swelling behind the eyeballs. The medicine teprotumumab (Tepezza) also may be used to control moderate to severe symptoms. If those medicines don’t ease symptoms, other medicines are sometimes used to treat thyroid eye disease. They include, tocilizumab (Actemra), rituximab (Rituxan) and mycophenolate mofetil (Cellcept).
In some cases, surgery may be needed to treat thyroid eye disease, including:
- Orbital decompression surgery. In this surgery, the bone between the eye socket and the sinuses is removed. This surgery can improve vision. It also gives the eyes more room, so they can go back to their usual position. There is a risk of complications with this surgery. If you have double vision before the surgery, it may not go away afterward. Some people develop double vision after the surgery.
- Eye muscle surgery. Sometimes scar tissue from thyroid eye disease can cause one or more eye muscles to be too short. This pulls the eyes out of alignment, causing double vision. Eye muscle surgery may correct double vision by cutting the muscle from the eyeball and attaching it again farther back.
The choice of treatment modality for hyperthyroidism caused by overproduction of thyroid hormones depends on the patient’s age, symptoms, comorbidities, and preference but also including what is the CAUSE of the hyperthyroidism determined by diagnostic tooling tests.