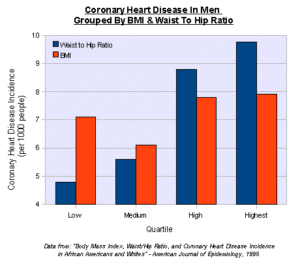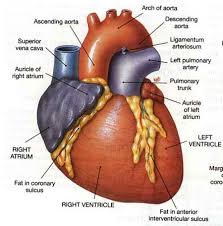
Heart disease is the leading cause of death for men and women in the United States. Every year, 1 in 4 deaths are caused by heart disease. The good news? Heart disease can often be prevented when people make healthy choices and manage their health conditions.
First let’s understand what occurs to the heart with bad health habits over a long period of time:
If you are eating too much for too long that consist of foods high in sodium, fat and cholesterol your vessels will narrow in size due to high B/P and blockages due to the high fat and cholesterol foods. By allowing high B/P you expose yourself to increasing the pressure in the vessels that increases your blood pressure called hypertension.
If you are also inactive you are at risk of obesity which puts stress on the heart and in time causing high B/P.
Constantly being exposed to a high B/P or HTN in the body this could cause the vessel to rupture (at the heart=possible heart attack, also called MI, at the brain=possible stroke, also called CVA with both on high occurrences in our population of the US.).
With bad habits (especially poor diet, inactive, and smoking) you can cause over time atherosclerosis=a blockage in the artery with the resolution surgery (from what they call a cardiac catheterization which commonly starts up your groin at the femoral artery or if the surgeon is having difficulty than the procedure is done in the arm leading up to the heart where the cardiac catheter or also called an angiogram which could lead to a angioplasty with possibly a stent is performed if there is a blockage. If they see the blockage (s) is very bad a CABG=coronary artery bypass=a 6hr plus operation where diversion of a vein from your leg or a stent (donor graft site) around the blockage is done. Smoking can lead to this. Also know smoking can cause your vessels to become brittle=arteriosclerosis.
Healthy Habits would impact a positive result for all people who have had this diagnosis CHF that is picked up or diagnosed early. Most important healthy habits can be a great as a PREVENTATIVE measure for people not diagnosed with any cardiac disease.
There are 4 things you have no control over heredity, age, sex, and race but healthy habits are sure to benefit you by keeping the odds down of you inheriting, help your age factor, and race a lot can be associated with eating cultural habits.
If you make the decision to live a life that’s healthy for your heart through proper eating, doing some exercise or activity with balancing rest in your busy schedule would be a great direction or taking the right step.
Wouldn’t you want less chance of heart disease or obesity or diabetes for yourself and for others throughout the nation including the future generations? Let’s build a stronger foundation regarding HEALTH in America=Prevention!
Communities, health professionals, and families can work together to create opportunities for people to make healthier choices. February is American Heart Month, a time when all people—especially women—are encouraged to focus on their cardiovascular health. This Heart Month, the Division for Heart Disease and Stroke Prevention (DHDSP) is encouraging women to listen to their hearts and speak up for their health.
Make a difference in your community: Spread the word about strategies for preventing heart disease and encourage people to live heart healthy lives.
How can American Heart Month make a difference? We can use this month to raise awareness about heart disease and how people can prevent it — both at home and in the community.
Here are just a few tips in prevention of heart disease:
• Encourage families to make small changes, like using spices to season their food instead of salt. • Motivate teachers and administrators to make physical activity a part of the school. This can help students start good habits early.
• Ask doctors and nurses to be leaders in their communities by speaking out about ways to prevent heart disease. How can you help spread the word? We’ve made it easier for you to make a difference. This toolkit is full of ideas to help you take action today. For example: Add information about living a heart healthy lifestyle to your newsletter. • Tweet about American Heart Month. • Host a community event where families can be active while learning about local health resources.
Also know smoking can cause your vessels to become brittle=arteriosclerosis. SO STOP SMOKING!
So prevent surgery like a cardiac cath or an artery bypass=a 6hr plus operation where diversion of a vein from your leg or a stent (donor graft site) around the blockage is done.
Healthy Habits would impact a positive result for all people who have had this diagnosis CHF that is picked up or diagnosed early. Most important healthy habits can be a great as a PREVENTATIVE measure for people not diagnosed with any cardiac disease.
There are 4 things you have no control over heredity, age, sex, and race but healthy habits are sure to benefit you by keeping the odds down of you inheriting, help your age factor, and race a lot can be associated with eating cultural habits.
If you make the decision to live a life that’s healthy for your heart through proper eating, doing some exercise or activity with balancing rest in your busy schedule would be a great direction or taking the right step.
Wouldn’t you want less chance of heart disease or obesity or diabetes for yourself and for others throughout the nation including the future generations? Let’s build a stronger foundation regarding HEALTH in America.
Take action: Be the cure! Join the American Heart Association’s national movement in support of healthier communities and healthier lives.