Charcot-Marie-Tooth disease (CMT) is one of the most common inherited neurological disorders, affecting approximately 1 in 2,500 people in the United States. The disease is named for the three physicians who first identified it in 1886 – Jean-Martin Charcot and Pierre Marie in Paris, France, and Howard Henry Tooth in Cambridge, England. CMT, also known as hereditary motor and sensory neuropathy (HMSN) or peroneal muscular atrophy, comprises a group of disorders that affect peripheral nerves. The peripheral nerves lie outside the brain and spinal cord and supply the muscles and sensory organs in the limbs. Disorders that affect the peripheral nerves are called peripheral neuropathies.
Causes of Charcot-Marie-Tooth disease?
A nerve cell communicates information to distant targets by sending electrical signals down a long, thin part of the cell called the axon. In order to increase the speed at which these electrical signals travel, the axon is insulated by myelin, which is produced by another type of cell called the Schwann cell. Myelin twists around the axon like a jelly-roll cake and prevents the loss of electrical signals. Without an intact axon and myelin sheath, peripheral nerve cells are unable to activate target muscles or relay sensory information from the limbs back to the brain.
CMT is caused by mutations in genes that produce proteins involved in the structure and function of either the peripheral nerve axon or the myelin sheath. Although different proteins are abnormal in different forms of CMT disease, all of the mutations affect the normal function of the peripheral nerves. Consequently, these nerves slowly degenerate and lose the ability to communicate with their distant targets. The degeneration of motor nerves results in muscle weakness and atrophy in the extremities (arms, legs, hands, or feet), and in some cases the degeneration of sensory nerves results in a reduced ability to feel heat, cold, and pain.
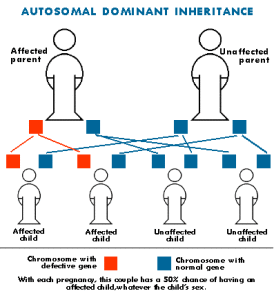
The gene mutations in CMT disease are usually inherited. Each of us normally possesses two copies of every gene, one inherited from each parent. Some forms of CMT are inherited in an autosomal dominant fashion, which means that only one copy of the abnormal gene is needed to cause the disease. Other forms of CMT are inherited in an autosomal recessive fashion, which means that both copies of the abnormal gene must be present to cause the disease. Still other forms of CMT are inherited in an X-linked fashion, which means that the abnormal gene is located on the X chromosome. The X and Y chromosomes determine an individual’s sex. Individuals with two X chromosomes are female and individuals with one X and one Y chromosome are male.
In rare cases the gene mutation causing CMT disease is a new mutation which occurs spontaneously in the individual’s genetic material and has not been passed down through the family. There are many forms of CMT disease, including CMT1, CMT2, CMT3, CMT4, and CMTX. CMT1, caused by abnormalities in the myelin sheath, has three main types.
CMT1A is an autosomal dominant disease that results from a duplication of the gene on chromosome 17 that carries the instructions for producing the peripheral myelin protein-22 (PMP-22). The PMP-22 protein is a critical component of the myelin sheath. Overexpression of this gene causes the structure and function of the myelin sheath to be abnormal. Patients experience weakness and atrophy of the muscles of the lower legs beginning in adolescence; later they experience hand weakness and sensory loss.
CMT1B is an autosomal dominant disease caused by mutations in the gene that carries the instructions for manufacturing the myelin protein zero (P0), which is another critical component of the myelin sheath.
CMT2 results from abnormalities in the axon of the peripheral nerve cell rather than the myelin sheath. It is less common than CMT1.
CMT3 or Dejerine-Sottas disease is a severe demyelinating neuropathy that begins in infancy. Infants have severe muscle atrophy, weakness, and sensory problems
CMT4 comprises several different subtypes of autosomal recessive demyelinating motor and sensory neuropathies. Individuals with CMT4 generally develop symptoms of leg weakness in childhood and by adolescence they may not be able to walk.
CMTX is caused by a point mutation in the connexin-32 gene on the X chromosome. Males who inherit one mutated gene from their mothers show moderate to severe symptoms of the disease beginning in late childhood or adolescence. Females who inherit one mutated gene from one parent and one normal gene from the other parent may develop mild symptoms in adolescence or later or may not develop symptoms of the disease at all.
How is Charcot-Marie-Tooth disease diagnosed?
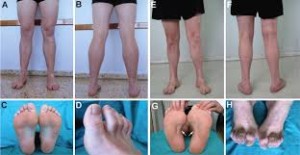
Diagnosis of CMT begins with a standard medical history, family history, and neurological examination. Individuals will be asked about the nature and duration of their symptoms and whether other family members have the disease. During the neurological examination a physician will look for evidence of muscle weakness in the individual’s arms, legs, hands, and feet, decreased muscle bulk, reduced tendon reflexes, and sensory loss. Doctors look for evidence of foot deformities, such as high arches, hammertoes, inverted heel, or flat feet. Other orthopedic problems, such as mild scoliosis or hip dysplasia, may also be present. A specific sign that may be found in people with CMT1 is nerve enlargement that may be felt or even seen through the skin. These enlarged nerves, called hypertrophic nerves, are caused by abnormally thickened myelin sheaths.
If CMT is suspected, the physician may order electrodiagnostic tests. This testing consists of two parts: nerve conduction studies and electromyography (EMG). During nerve conduction studies, electrodes are placed on the skin over a peripheral motor or sensory nerve. These electrodes produce a small electric shock that may cause mild discomfort. This electrical impulse stimulates sensory and motor nerves and provides quantifiable information that the doctor can use to arrive at a diagnosis. EMG involves inserting a needle electrode through the skin to measure the bioelectrical activity of muscles. Specific abnormalities in the readings signify axon degeneration. EMG may be useful in further characterizing the distribution and severity of peripheral nerve involvement.
Genetic testing is available for some types of CMT and results are usually enough to confirm a diagnosis. In addition, genetic counseling is available to assist individuals in understanding their condition and plan for the future.
If all the diagnostic work-up in inconclusive or genetic testing comes back negative, a neurologist may perform a nerve biopsy to confirm the diagnosis. A nerve biopsy involves removing a small piece of peripheral nerve through an incision in the skin. This is most often done by removing a piece of the nerve that runs down the calf of the leg. The nerve is then examined under a microscope.
The treatment of CMT:
There is no cure for CMT, but physical therapy, occupational therapy, braces and other orthopedic devices, and even orthopedic surgery can help individuals cope with the disabling symptoms of the disease. In addition, pain-killing drugs can be prescribed for individuals who have severe pain.
Physical and occupational therapy, the preferred treatment for CMT, involves muscle strength training, muscle and ligament stretching, stamina training, and moderate aerobic exercise. Most therapists recommend a specialized treatment program designed with the approval of the person’s physician to fit individual abilities and needs. Therapists also suggest entering into a treatment program early; muscle strengthening may delay or reduce muscle atrophy, so strength training is most useful if it begins before nerve degeneration and muscle weakness progress to the point of disability.
Stretching may prevent or reduce joint deformities that result from uneven muscle pull on bones. Exercises to help build stamina or increase endurance will help prevent the fatigue that results from performing everyday activities that require strength and mobility. Moderate aerobic activity can help to maintain cardiovascular fitness and overall health. Most therapists recommend low-impact or no-impact exercises, such as biking or swimming, rather than activities such as walking or jogging, which may put stress on fragile muscles and joints.
Many CMT patients require ankle braces and other orthopedic devices to maintain everyday mobility and prevent injury. Ankle braces can help prevent ankle sprains by providing support and stability during activities such as walking or climbing stairs. High-top shoes or boots can also provide support for weak ankles. Thumb splints can help with hand weakness and loss of fine motor skills. Assistive devices should be used before disability sets in because the devices may prevent muscle strain and reduce muscle weakening. Some individuals with CMT may decide to have orthopedic surgery to reverse foot and joint deformities.
The National Institute of Neurology Disorders and Stroke supports research on CMT and other peripheral neuropathies in an effort to learn how to better treat, prevent, and even cure these disorders.